Development of Chemical Strategies
to Study Neurobiological Processes
|
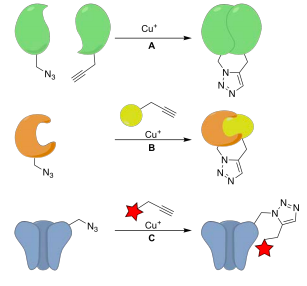
Our research centers on the design and synthesis of molecular probes to monitor and influence dynamic processes in the brain. Specifically, synthetic organic chemistry is integrated to a multidisciplinary approach to elucidate the molecular mechanisms of neuroglial communication, synapse elimination, and neurogenesis. The research programs outlined below have in common the selective modification of proteins to investigate their role in neuronal physiology.
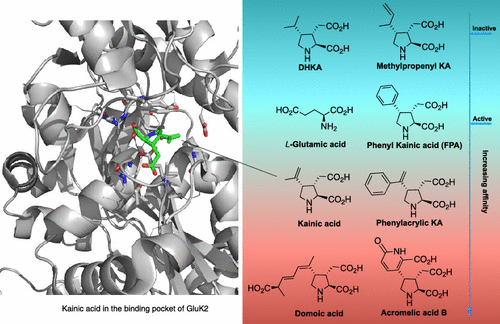
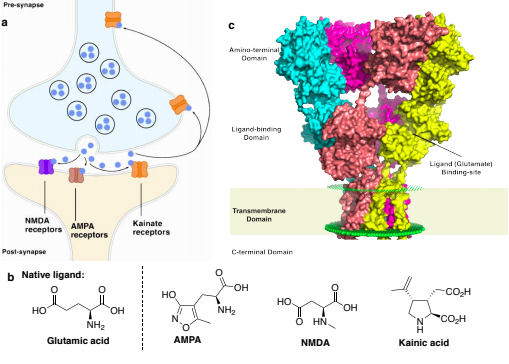
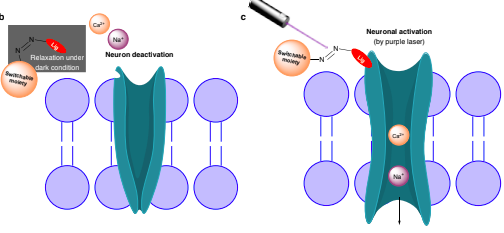
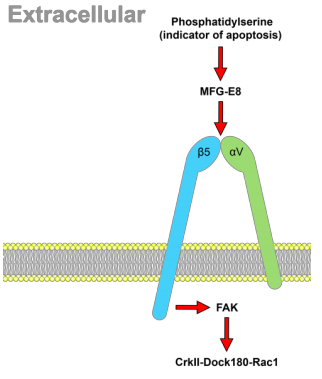
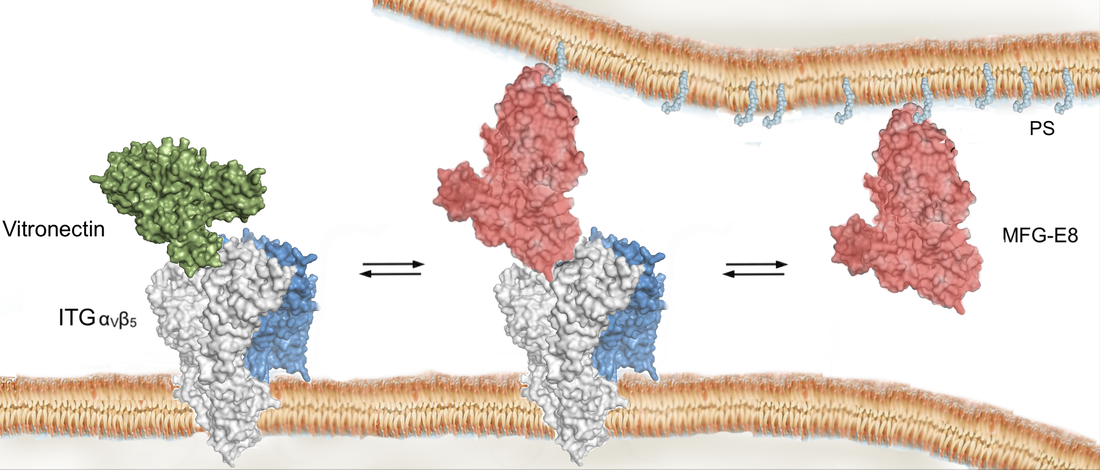
The long-term goal of our efforts is to create tools to understand the mysterious function of astrocytes in cognitive and degenerative diseases. In the brain, astrocytes make crucial contributions to the formation, operation and adaptation of neural circuitry. As neurobiology evolves into a truly molecular science, organic chemists are uniquely positioned to make significant contributions to the field. 1. Synthesis of Modified Natural Products to Study the Calcium Signaling Cascade in Glial Cells The calcium signaling cascade in a set of glial cells known as astrocytes regulates the coherent firing of neurons by reinforcing neurotransmitters release at synapses in the brain. We exploit natural products displaying exquisite selectivity for Ca2+ ion channels to elucidate the function of Ca2+-release events in astrocytes. Domoic acid, and Kainic acid are examples of molecules that can be modified with chemical reporter tags to simultaneously control and monitor Ca2+ cellular signaling (e.g., cellular entry, signal amplification, and intracellular depletion). The labeled molecules are prepared by total synthesis or semisynthesis. We evaluate their interaction with target proteins expressed in model cells. Then, we use the molecules to characterize the native dynamics of Ca2+ signaling events in astrocytes using combined microscopy imaging and electrophysiology techniques. Finally, we will apply the modified natural products to deconvolute the microscopic events underpinning the communication between neurons and glia. While we choose to study astrocytes for the exciting questions they pose, the ubiquity of Ca2+ion channels will make these probes broadly applicable to the study of Ca2+ signaling in other tissues (e.g., heart, muscles). 2. Ligand–Directed Chemical Ligation of Lysines: Labeling of Functional Proteins in Living Systems The introduction of non-genetically encoded synthetic probes into specific proteins is one of the main challenges in Chemical Biology. Our goal is to develop a general strategy to label proteins in their native environment based on their intrinsic structural features. Lysines residues are latent nucleophiles commonly found at the surface of proteins, yet no general method exists for their selective ligation. Our approach to lysine labeling relies on using affinity ligands to favour entropy and to mitigate unfavourable kinetics of intermolecular reactions. We capitalize on new molecular reagents designed to effect site-directed, tag-release modification of lysine residues under physiological conditions. Upon chemoselective reaction with a lysine, the ligand is cleaved to restore a labeled, functional protein. We currently study protein-protein interactions using fluorescent probes and super-resolution imaging with collaborators. This chemical approach provides powerful tools to study proteins in living systems and will impact a range of interdisciplinary contexts. It will offer an invaluable alternative to current biology protocols using genetic modification of proteins. 3. Investigating the Mechanisms of Synapse Elimination Memory loss occurs when synaptic connections are broken. Yet, synapse elimination also takes place during the normal learning process... What causes synapse elimination? Can it be prevented? or reversed? This important phenomenon is poorly understood due to the lack of means to study its mechanism at the molecular level. Our traceless chemical ligation strategy enables the labeling of target proteins while maintaining their innate function, thereby alleviating functional perturbations associated with common molecular biology techniques. It is thus perfectly suited to deliver biophysical reporter probes and to characterize the interaction of proteins in real time, in live cells. In our quest to understand how synaptic connections get eliminated in the brain, we have identified a set of proteins involved in cellular signaling between neurons and astrocytes. One such protein found only in astrocytes is integrin αVβ5 (ITGαVβ5). Whereas members of the large family of integrins have attracted a lot of attention for their potential role in cancer, the exact function of ITGαVβ5 in the brain remains unsolved. This is in part due to the lack of tools available to study signaling proteins in living system. Evidence suggests that ITGαVβ5 may be involved in synapse elimination by triggering endocytosis processes. Our initial objective is to develop a reliable method to label ITGαVβ5 and study how its behaviour changes in its native milieu with regard to its associated signaling proteins. As entry point into the metabolic cascade, we are characterizing the dynamic function of two proteins, ITGαVβ5 and MFG-E8. The structural information about ITGαVβ5 and MFG-E8 makes them ideal candidates for the optimization of our traceless labeling method. Combining these techniques with methods of molecular biology will lead to unprecedented levels of characterization of complex signaling pathways involved in synapse elimination and shed light on neuron–astrocyte communication. |